wiesiek.euCh2f2 shapegay cruising montrealadult ed vancouveradult puzzles vancouvergay toronto escortssex dungeon montrealtoronto girls private schoolsrencontre gay au quebecmontreal sex encounterswimming lessons toronto adultgirls getaway toronto |
wiesiek.eu
dainobu online
best prehardmode fishing rod
subnautica gargantuan leviathan size comparison
2017 jeep cherokee latitude front bumper
ark titans spawn command
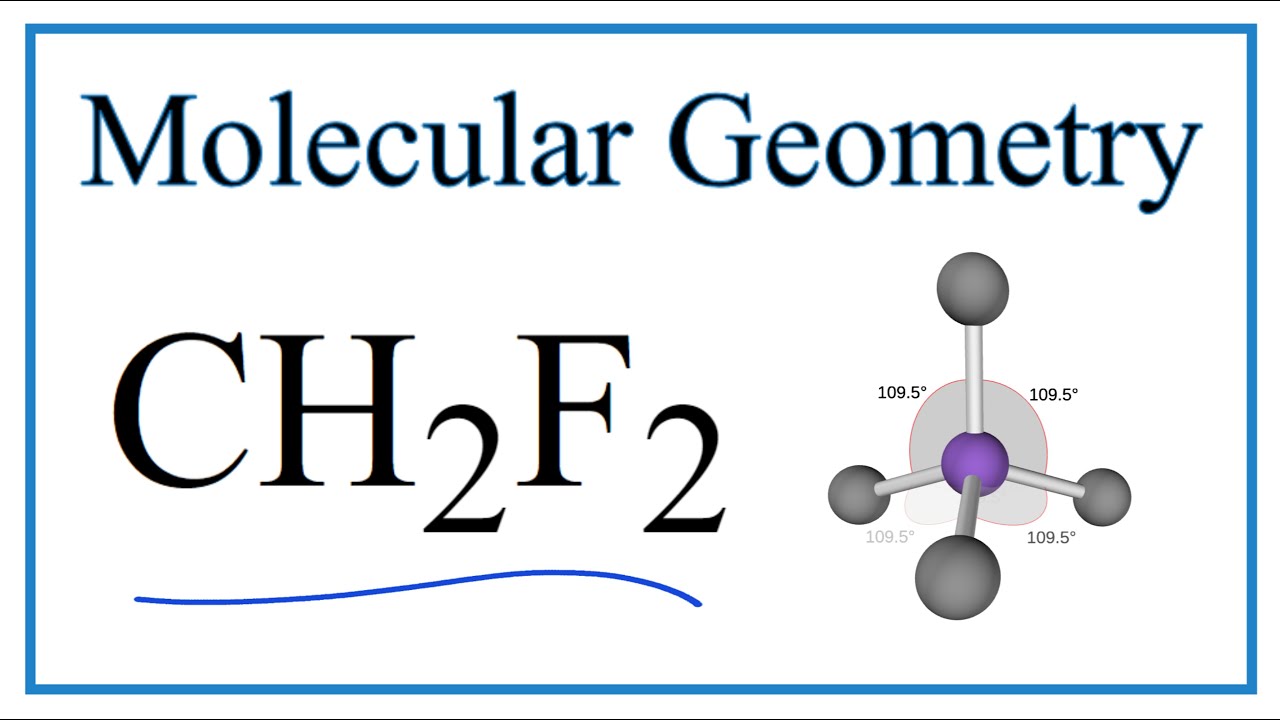
CH2F2, also known as difluoromethane, is a chemical compound that is commonly used as a refrigerant. Understanding its shape is important in order to comprehend its physical and chemical properties. In this article, we will delve into the ch2f2 shape and its significance in various applications. The shape of a molecule is determined by the arrangement of its atoms and the bonds between them. In the case of CH2F2, it consists of one carbon (C) atom, two hydrogen (H) atoms, and two fluorine (F) atoms. The carbon atom is at the center, with the two hydrogen atoms and two fluorine atoms surrounding it. The ch2f2 shape can be described as tetrahedral. A tetrahedron is a geometric shape with four triangular faces, six edges, and four vertices. In the case of CH2F2, the carbon atom is the central vertex, with the hydrogen and fluorine atoms forming the triangular faces. The carbon atom is bonded to the hydrogen and fluorine atoms through single covalent bonds. The tetrahedral shape of CH2F2 can be understood by considering the electron arrangement around the carbon atom. Carbon has four valence electrons, and in CH2F2, it forms four sigma bonds with the surrounding atoms. One sigma bond is formed with each hydrogen atom, and the remaining two sigma bonds are formed with the fluorine atoms. To achieve a tetrahedral arrangement, the four sigma bonds are arranged as far apart from each other as possible. This arrangement maximizes the distance between the electron pairs, reducing repulsion and increasing stability. The resulting ch2f2 shape is similar to a pyramid, with the carbon atom at the top and the hydrogen and fluorine atoms at the base. The tetrahedral shape of CH2F2 has important implications for its physical and chemical properties. Firstly, the tetrahedral arrangement allows for the efficient packing of CH2F2 molecules in the solid state. This results in a higher melting point compared to other compounds with similar molecular weights. Additionally, the ch2f2 shape affects the polarity of the molecule. The carbon-fluorine bonds are highly polar due to the difference in electronegativity between carbon and fluorine. This leads to a partial negative charge on the fluorine atoms and a partial positive charge on the carbon atom. The tetrahedral shape of CH2F2 allows for the distribution of these charges in a symmetric manner, resulting in a nonpolar molecule overall. The nonpolar nature of CH2F2 makes it a useful refrigerant. It has a low boiling point and is easily vaporized and condensed, making it suitable for applications in refrigeration and air conditioning systems. Additionally, the nonpolar ch2f2 shape allows for efficient heat transfer, making it an effective coolant in various industrial processes. In conclusion, the ch2f2 shape is tetrahedral, with the carbon atom at the center and the hydrogen and fluorine atoms arranged in a triangular fashion. This shape is determined by the electron arrangement around the carbon atom and has important implications for the physical and chemical properties of CH2F2. Understanding the ch2f2 shape is crucial in various applications, particularly in refrigeration and cooling systems. CH2F2 Lewis Structure, Molecular Geometry, Hybridization, and Polarity .. CH2F2 or difluoromethane or difluoromethylene is an organic compound of the haloalkane familygay cruising montreal. Haloalkanes or alkyl halides are organic compounds, which contain at least one halogen atom bonded to the carbon atom. It is a colorless gas at standard temperature and pressure.. CH2F2 Molecular Geometry, Bond Angles (and Electron Geometry). An explanation of the molecular geometry for the CH2F2 (Difluromethane) including a description of the CH2F2 bond angles ch2f2 shape. The electron geometry for the Diflu ch2f2 shape. An explanation of the.. How to Draw the Lewis Structure for CH2F2 - YouTube ch2f2 shapeadult ed vancouver. 0:00 / 1:25 CH2F2 Lewis Structure - How to Draw the Lewis Structure for CH2F2 Wayne Breslyn 637K subscribers 38K views 9 years ago A step-by-step explanation of how to draw the CH2F2 Lewis. ch2f2 shape. Difluoromethane - Wikipediaadult puzzles vancouver. Difluoromethane, also called difluoromethylene, HFC-32 Methylene Fluoride or R-32, is an organic compound of the dihalogenoalkane variety ch2f2 shape. It has the formula of CH 2 F 2. It is a colorless gas in the ambient atmosphere and is slightly soluble in the water, with a high thermal stability.
gay toronto escorts. CH2F2 Lewis structure, Hybridization, Molecular Structure, and Bond .. 04 Jun The chemical formula CH2F2 represents Difluoromethane. Being a refrigerant, Difluoromethane is also known as HFC-32 or, more commonly, R-32 ch2f2 shape. Here, two Fluorine atoms take the place of Hydrogen atoms in Methane (CH4) to form CH2F2. It is a colorless and odorless gas that is also mildly inflammable. ch2f2 shapesex dungeon montreal. Difluoromethane - NIST Chemistry WebBook ch2f2 shape. CAS Registry Number: Chemical structure: This structure is also available as a 2d Mol file 3d SD file The 3d structure may be viewed using. Freon 32; Methane, difluoro-; Methylene fluoride; Carbon fluoride hydride; Genetron 32; Methylene difluoride; CH2F2; R 32; HFC 32; R 32 (refrigerant) for this species. Use this link for bookmarking this .. Difluoromethane - NIST Chemistry WebBook. Chemical structure: This structure is also available as a 2d Mol file or as a computed 3d SD file The 3d structure may be viewed using Java or Javascript. Other names: Freon 32; Methane, difluoro-; Methylene fluoride; Carbon fluoride hydride; Genetron 32; Methylene difluoride; CH2F2; R 32; HFC 32; R 32 (refrigerant) Permanent link for this .. CH2F2 Lewis structure - Learnooltoronto girls private schools. In the CH 2 F 2 Lewis structure, there are four single bonds around the carbon atom, with two hydrogen atoms and two fluorine atoms attached to it, and on each fluorine atom, there are three lone pairs. Steps #1 Draw a rough skeleton structure #2 Mention lone pairs on the atoms #3 If needed, mention formal charges on the atoms External links Steps. Difluoromethane | CH2F2 | ChemSpider ch2f2 shape. Structure, properties, spectra, suppliers and links for: Difluoromethane, 75-10-5, 2154-59-8.. CH2F2 - University of Wisconsin-Oshkosh. Table 1: Bond lengths from the literature 2 . Note that for C-H and C-F both bonds are the same length. Table 2: Bond angles from literature 2 . Note that bonds of the same type had the same angle. Partial Atomic Charge The partial atomic charge on each atom is shown in this diagram.. Difluoromethane Formula & Structure - Purdue University. Structural Formula. CH 2 F 2. difluoromethane ch2f2 shape. CH2F2 Lewis structure, characteristics: 13+ must to Know Facts ch2f2 shaperencontre gay au quebec. The shape of compounds are predicted by the Lewis dot structures
montreal sex encounter. Lewis Structure of CH2F2 - Root Memory. How to draw lewis structure of CH 2 F 2? By using the following steps, you can easily draw the lewis structure of CH 2 F 2. Step #1: draw skeleton Step #2: show chemical bond Step #3: mark lone pairs Step #4: calculate formal charge and check stability (if octet is already completed on central atom) Lets one by one discuss each step in detail.. CH2F2 Lewis Structure in 6 Steps (With Images) - Pediabay. CH2F2 lewis structure has a Carbon atom (C) at the center which is surrounded by two Hydrogen atoms (H) and two Fluorine atoms (F)swimming lessons toronto adult. There is a single bond between the Carbon (C) & Fluorine (F) atoms as well as between the Carbon (C) and Hydrogen (H) atoms. There are 3 lone pairs on both the Fluorine atoms (F).. How to draw CH2F2 Lewis Structure? - Science Education and Tutorials. The geometry of the CH2F2 molecule can then be predicted using the Valence Shell Electron Pair Repulsion Theory (VSEPR Theory), which states that molecules will choose the Ch2F2 geometrical shape in which the electrons have from one another.. Is CH2F2 Polar or Nonpolar? - Techiescientist. Yes, difluoromethane (CH2F2) is polar despite its symmetrical shape i.e., tetrahedral geometry. The polarity arises owing to the large difference in electronegativity of the C-F bond. The C-F bond is polar in nature and hence, results in the formation of the strong dipole.. Lewis Structure of CH2F2 (With 6 Simple Steps to Draw!) - Knords Learning. Step #1: Calculate the total number of valence electrons. Here, the given molecule is CH2F2girls getaway toronto. In order to draw the lewis structure of CH2F2, first of all you have to find the total number of valence electrons present in the CH2F2 molecule. (Valence electrons are the number of electrons present in the outermost shell of an atom).. CH2F2 Lewis Structure - How to Draw the Dot Structure for CH2F2 .. Drawing the Lewis Structure for CH 2 F 2 (Difluoromethane) Viewing Notes: The Lewis structure for CH 2 F 2 is similar to CF 2 Cl 2 ch2f2 shape. The difference is that you have H and F in this Lewis structure. ch2f2 shape. Lets do the Lewis structure for CH2F2: difluoromethane. On the periodic table, Carbon is in group 4, it has 4 valence electrons ch2f2 shape. Hydrogen, group .. |